Protease-Releasable Masking Technology Designed to Safety Harness the Power of Potent Immune Modulators Through Preferential Activation in the Tumor Microenvironment by Tumor-Associated Proteases
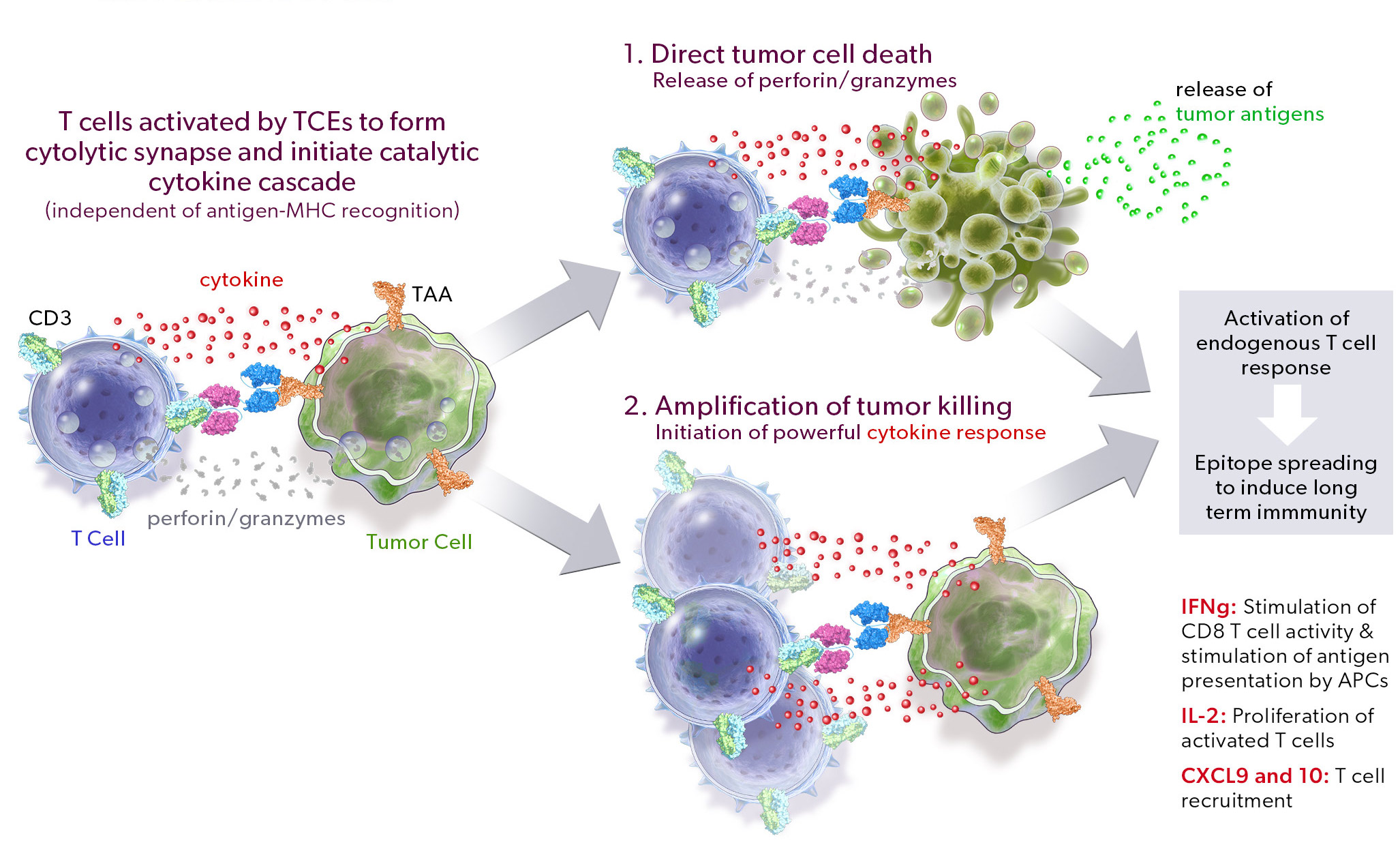
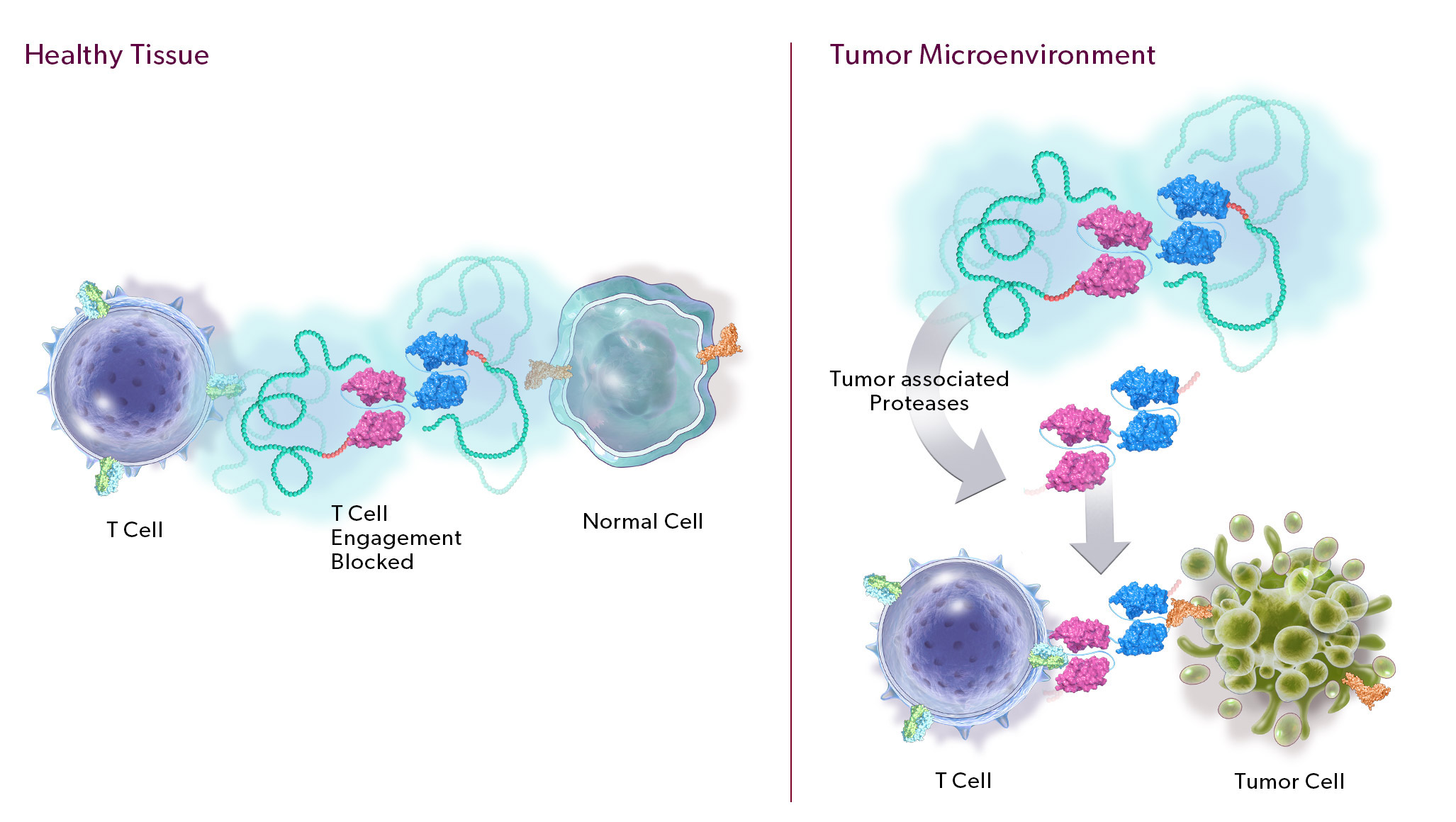
Our PRO-XTEN technology exploits the intrinsically high protease activity of the tumor microenvironment to preferentially unmask and activate our product candidates in the tumor as compared to healthy tissues, thereby mitigating off-tumor toxicity. Importantly, we already have clinical validation of the key properties of our technology, including half-life extension, masking, protease-driven unmasking, and low immunogenicity, as over 200 patients have been treated with product candidates developed by partners who have licensed our technology outside of oncology. We are now using this validated technology in a new way to create our proprietary oncology pipeline.
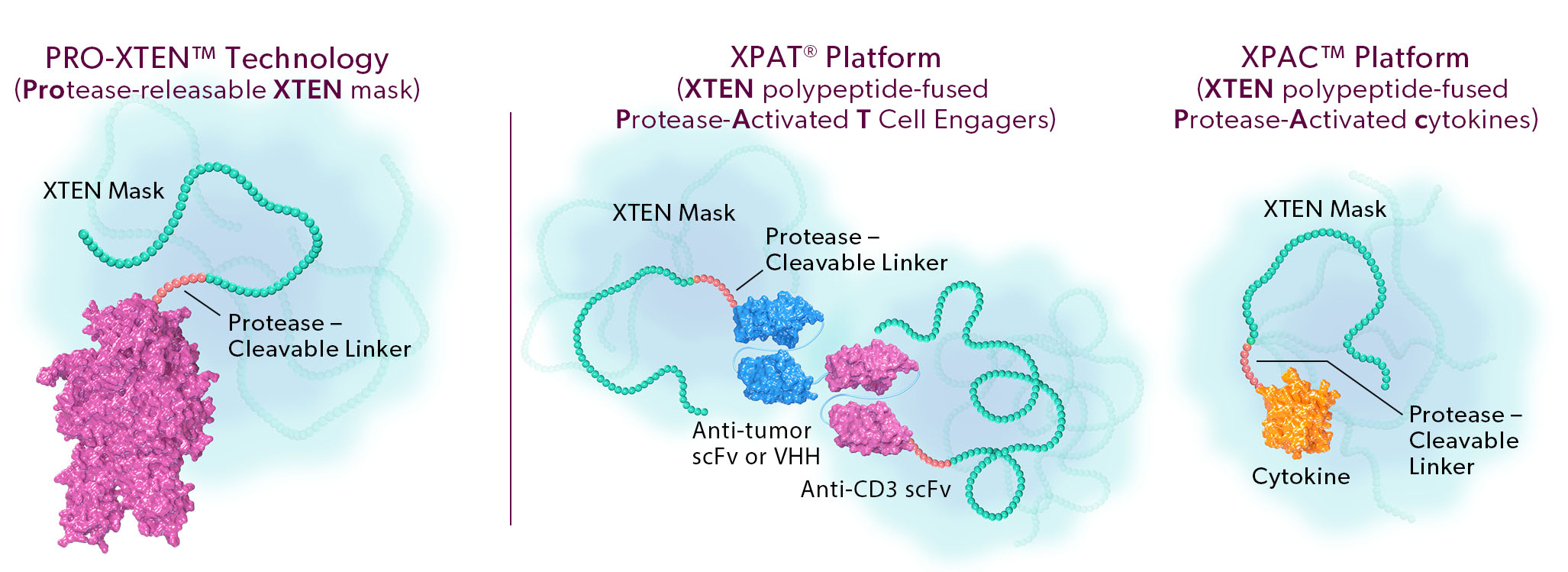
The two proprietary components of this technology are as follows:
- Universal, Tunable XTEN Mask. Our XTEN mask is a polypeptide that serves a dual purpose: 1) it acts as a universal, tunable mask, and 2) it provides half-life extension, which we believe can enable more convenient dosing for patients. Our XTEN masks drive a next generation masking strategy that can be applied universally to a broad array of protein therapeutic modalities. In contrast to first-generation masks, our novel XTEN masks do not require customized and specific binding to a functional region of the drug, instead acting as spatial shields that occupy space around a functional region. This mechanism of spatial shielding allows XTEN masks to be universally applicable regardless of the sequence or characteristics of the protein being masked. The strength of the masking can be fine-tuned by adjusting the length, number, and position of XTEN masks on a protein.
- Protease-Cleavable Linker. Our protease-cleavable linker enables preferential unmasking and drug activation in the tumor microenvironment. We designed it to be universally cleaved across tumor types to drive efficacy, yet remain largely intact in systemic circulation and in healthy tissues to enable safety.